While primary (non-rechargeable) batteries are ubiquitous in modern society, often overlooked are the differences between consumer-grade and industrial-grade batteries.
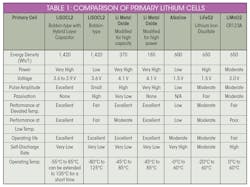
On the consumer side, many applications require batteries that can deliver higher discharge rates of energy, resulting in very short operating life (alkaline). Consumer-grade lithium batteries can deliver medium to high discharge rates of energy with short to medium operating life. These primary lithium chemistries include iron disulfate (LiFeS2), lithium manganese dioxide (LiMNO2), etc. (Table 1).
On the opposite side of the spectrum are a growing number of low-power remote wireless devices that use very small amounts of energy, measurable in microamps of average current. Many of these devices are connected to the Industrial Internet of Things (IIoT), supporting applications that require decades of maintenance-free operation without battery replacement.
Only Certain Batteries Can Operate for Decades in Extreme Environments
Lithium-based batteries have high intrinsic negative potential, exceeding that of all other metals, with an operating current voltage (OCV) ranging from 2.7 to 3.6 V. Lithium batteries are also non-aqueous, with the absence of water enabling them to endure extreme temperatures without freezing.
Among the available chemistries, bobbin-type lithium-thionyl-chloride (LiSOCl2) cells are overwhelmingly preferred for remote wireless applications in extreme environments, where average current discharge is measurable in microamps. Bobbin-type LiSOCl2 batteries feature the highest capacity and highest energy density of any lithium chemistry, along with an extremely low annual self-discharge rate (less than 1% per year), enabling certain low-power devices to operate for up to 40 years.
LiSOCl2 batteries have the highest capacity and highest energy density of any lithium chemistry, as well as featuring an extremely low annual self-discharge rate.
This chemistry also features the widest temperature range (−80 to 125°C), and a glass-to-metal hermetic seal that helps prevent battery leakage. Typical applications include AMR/AMI metering, M2M, SCADA, tank-level monitoring, asset tracking, and environmental sensors, to name a few.
Passivation Effect Reduces Battery Self-Discharge
All batteries suffer from self-discharge, where cell capacity is exhausted even when the battery isn’t connected to an external load.
Controlled passivation, which is unique to bobbin-type LiSOCl2 batteries, can greatly reduce self-discharge. Passivation occurs when a thin film of lithium chloride (LiCl) forms on the surface of the lithium anode, thus impeding the chemical reactions that result in battery self-discharge. When a load is placed on the cell, the passivation layer causes high initial resistance, resulting in a temporary drop in cell voltage until the discharge reaction slowly removes the passivation layer—a process that repeats itself every time the load is removed.
Various factors can influence passivation, including the current capacity of the cell, length of storage, storage temperature, discharge temperature, and prior discharge conditions. Partially discharging a cell and then removing the load increases the amount of passivation relative to when the cell was new.
Passivation involves tradeoffs, too. It’s necessary to reduce battery self-discharge rate, but too much of it can block energy flow.
Bobbin-type LiSOCl2 batteries can also be designed with lower amounts of passivation to deliver medium energy-flow rates and higher self-discharge, resulting in a shorter lifespan of 10-15 years.
In addition, battery self-discharge is impacted by the quality of the raw materials and the way the battery is manufactured. For example, a lower quality bobbin-type LiSOCl2 battery designed for ultra-long life can lose 3% of its normal capacity each year to self-discharge. Thus, 30% of its initial capacity is exhausted every 10 years, making 40-year battery life impossible to achieve. By contrast, a superior quality bobbin-type LiSOCl2 battery can feature a self-discharge rate of 0.7% per year, retaining 93% of its original capacity after 10 years, enabling a 40-year marathon.
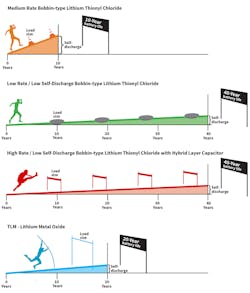
The Race Analogy: Separating the Sprinters from the Marathoners
The Distance is equivalent to the battery/device operating life. The longer the runner can run, the more years a device will be able to operate.
The Incline is equivalent to the battery self-discharge. The higher the self-discharge rate, the larger the incline. Just as the incline draws more power from the runner and shortens his run, the self-discharge of the battery reduces the availability of useful power for device operation and lowers the operating life.
Hurdles are equivalent to pulses. The higher the hurdle, or obstacle, the higher the pulse ability of the battery.
Generally, applications that require very high energy drain rates and high pulses, such as medical power tools, with average current measurable in amps, may be well-suited for lithium metal-oxide batteries. Applications requiring moderate rates of discharge, measurable in milliamps to amps, such as powering a flashlight or consumer toy for limited operating times, may be best suited for alkaline, LiFeS2, and LiMNO2 batteries that are able to deliver medium pulses.
Ultra-long-life, low-drain applications, with average current measurable in microamps, including many remote wireless sensors, require the use of standard bobbin-type LiSOCl2 batteries that can run marathons due to their very low self-discharge rates. However, they’re not designed to deliver high pulses due to their low rate design.
For ultra-long-life applications that require periodic high pulses of energy to power two-way wireless communications, standard bobbin-type LiSOCl2 batteries must be modified using a patented hybrid layer capacitor (HLC). The standard bobbin-type LiSOCl2 cell delivers low daily background current (to continue running marathons) while the HLC delivers periodic high pulses (for steeple jumping). The patented HLC also features a special end-of-life voltage plateau that can be interpreted to deliver low-battery status alerts.
Supercapacitors deliver high pulses electrostatically rather than chemically. Supercapacitors are often used in consumer electronics where environmental conditions are moderate (indoor track). However, supercapacitors are rarely used in industrial applications (cross-country running) due to inherent drawbacks such as short-duration power, linear discharge qualities that prevent use of all available energy, low capacity, low energy density, and high annual self-discharge rates (up to 60% per year). Supercapacitors linked in series also require the use of cell-balancing circuits, which adds to their cost and bulkiness and consumes additional energy, increasing their self-discharge rate even further.
Short-Term Tests Fail to Simulate a Marathon
Long-term battery performance is difficult to simulate with short-term tests, so appropriate methods must be used to deliver verifiable results that predict long-term performance. Proven techniques include:
- Long-term laboratory testing: The ideal way to monitor battery self-discharge is to continually test batteries over time under various conditions, covering almost every possible scenario. The accumulated data points can measure cell size, temperature, load size, etc., resulting in a vast and ever-growing database that enables highly accurate predictive models.
- Accelerated testing: The Arrhenius equation (involving a two-fold increase of reaction rate for every 10°C rise in temperature) can be helpful in shortening the time it takes to simulate long-term operation. Arrhenius tests are run at 72°C, equivalent to about 32 times the theoretical lifetime of battery at 22°C. However, short-term tests using the Arrhenius method tend to show inaccurate results.
- Calorimeter testing: A highly accurate test method is to measure actual heat-energy losses using a state-of-the-art microcalorimeter, which can detect energy dissipation down to the 0.1-W level.
Heat energy is generated three ways: entropy change, often referred to as reversible heat; cell over-protection, often referred to as irreversible heat; and chemical reactions, including self-discharge reactions that affect cell capacity, and side reactions that don’t affect cell capacity.
Calorimeter testing can measure losses in battery capacity caused during long-term storage or operation (including self-discharge), which is typically computed using thermodynamic equations and cell-voltage considerations. Accurate long-term tests require that the batteries be stabilized for one year prior to testing, as self-discharge during the first year tends to be higher than subsequent years. Other test methods include:
- Lithium titration: In special circumstances, where sufficient long-term data points may not be available (i.e., exposure to extreme temperatures, prolonged high current pulses, short lifetime applications, etc.), lithium titration can be used to measure available cell capacity. The battery is cut open, and titration is used to dissolve the remaining lithium. The higher the self-discharge rate, the less amount of lithium will remain in the cell.
- Field results: Lab tests create theoretical models that can be verified using actual results from the field. For example, Tadiran works closely with its customers to randomly test batteries taken from long-term deployments to demonstrate in real-life how long-term exposure to extreme temperatures can affect battery self-discharge.
Another useful indicator is to calculate the number of failures in time (FITs), measurable in billions of device operating hours for devices in the field. Tadiran batteries, for instance, achieve FIT rates ranging between 5 and 20 batteries per billion, which is extremely low compared to the industry average.
How to Choose an Industrial-Grade Battery
Short-term tests generally under-represent the true effects of passivation and long-term exposure to extreme temperatures. If extended battery life is a critical requirement, then thorough due diligence must be performed to properly evaluate competing batteries. Complete verification requires fully documented long-term test results, in-field performance data from similar applications, and customer references.
Obtaining verifiable test data is essential to critical applications such as meter transmitter units (MTUs) used in AMR/AMI utility metering, as a large-scale battery failure can disrupt customer billing systems and disable remote service startup and shutoff capabilities. The possibility of such wide-scale chaos could force a utility to prematurely invest millions of dollars to replace batteries early so as not to jeopardize data integrity.
Other factors need to be considered when specifying an industrial-grade lithium battery, including the amount of current consumed in active mode (along with the size, duration, and frequency of pulses); energy consumed in standby or sleep mode (the base current); storage time (as normal self-discharge during storage diminishes capacity); expected temperatures (including during storage and in-field operation); equipment cutoff voltage (as battery capacity is exhausted, or in extreme temperatures, voltage can drop to a point too low for the sensor to operate).
If a remote wireless application draws milliamps of average current, this may be enough to prematurely exhaust a primary battery. In some cases, the application may better suited for an energy-harvesting device in conjunction with a rechargeable lithium-ion (Li-ion) battery to store the harvested energy.
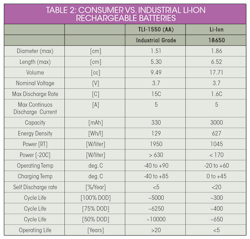
CattleWatch hub collars have built-in photovoltaic panels that harvest energy, which is stored in industrial-grade Li-ion rechargeable batteries that can deliver the high pulses needed to ensure satellite-based real-time communications between the in-herd mesh network and the rancher.
Consumer-grade rechargeable Li-ion batteries can operate for up to five years and 500 recharge cycles, within a moderate temperature range, and no ability to deliver high pulses (an easy 10K jog at moderate temperatures with no big hills). By contrast, industrial-grade rechargeable Li-ion batteries can operate for up 20 years and 5,000 full recharge cycles (half-marathoners). Other benefits of industrial grade Li-ion batteries include the ability to be charged and discharged at extreme temperatures, and the ability to deliver up to 15-A pulses to power two-way wireless communications (Table 2).
Every application is unique, so it’s important to specify the right battery, be it a sprinter (high discharge potential); a medium distance runner (moderate to high discharge rate with fairly low self-discharge); or a 40-year marathoner (including elite runners who can jump periodic high pulse hurdles).
Sol Jacobs is VP & General Manager of Tadiran Batteries.
About the Author
Sol Jacobs
VP & General Manager
Sol Jacobs, VP and General Manager for Tadiran Batteries, has over 30 years of experience in powering remote devices. His educational background includes a BS in Engineering and an MBA.